In daily life, our standards for judging water quality are quite simple. If it looks clear and tastes odorless, then it's considered good water. However, if we were to inject this seemingly clean tap water—or even ordinary filtered water—directly into the high-temperature, high-pressure boilers of a modern power plant, the consequences would be disastrous.
For an industrial boiler operating under heavy load, water is not just a flowing liquid; it's more like a precision structural component. This is why industry standards mandate the use of ultrapure water (also often called demineralized or deionized water). This is not for the sake of formality or pursuing some kind of aesthetic ideal, but because in extreme physical environments, the purity of the water directly determines whether the factory can operate safely and profitably, or face huge losses or even explosion accidents.
Why are the standards for water used in boilers hundreds of times stricter than those for human drinking water? The answer lies in the microscopic world invisible to the naked eye.
Take a glass of ordinary tap water and examine it under a microscope or with chemical test strips, and you'll find that it's actually an extremely complex "chemical soup." It contains dissolved salts (calcium, magnesium, sodium), dissolved gases (oxygen, carbon dioxide), and colloidal silica.
At normal temperature and pressure, these impurities are docile and harmless. However, once they enter the boiler—in this extreme environment where temperatures exceed 500°C and pressures reach tens or even hundreds of atmospheres—they undergo violent thermochemical reactions, transforming into three major killers that destroy equipment: scaling, corrosion, and steam contamination.
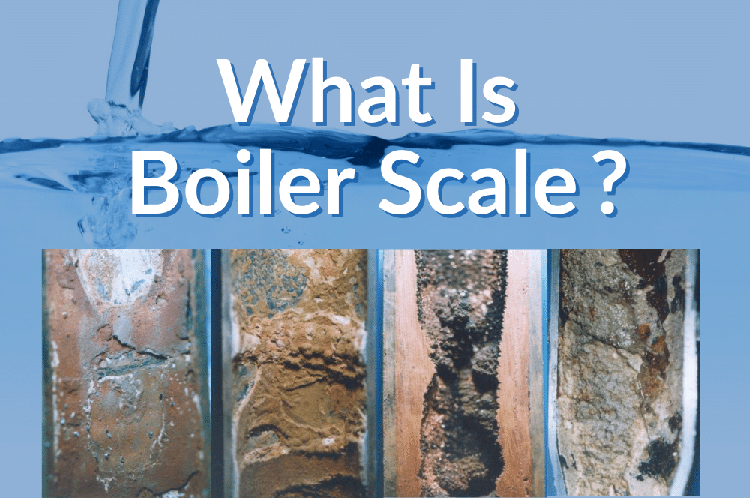
The first crisis triggered when ordinary water enters the boiler is scaling. This is similar to the limescale that builds up in our kettles after prolonged use, but in industrial boilers, this phenomenon is infinitely magnified and extremely dangerous.
As the water evaporates, the concentration of calcium and magnesium ions in the water rapidly saturates. They precipitate out and firmly adhere to the inner walls of the boiler pipes, forming a dense, hard, rock-like substance.
Do not underestimate this layer of scale. Data shows that the thermal conductivity of scale is usually only a few hundredths or even thousandths of that of steel. This means that even a layer of scale the thickness of a coin on the pipe wall can block heat as effectively as a thick layer of insulation.
What are the consequences?
The flames in the furnace work hard to heat the water, but the heat is blocked by the scale and cannot be transferred to the water inside the pipes. The heat accumulates on the metal of the pipe wall, causing the metal temperature to skyrocket, quickly exceeding the yield strength of the steel. Under immense internal pressure, the red-hot steel pipe bulges like a balloon and eventually bursts. In my industry observations, this "pipe burst and shutdown" caused by poor water quality management results in astronomical economic losses every day, not to mention the potential risks to personnel safety.

If scaling is a hard barrier, then corrosion is a silent disintegration.
Dissolved oxygen in water is the number one enemy of boiler metal. Under the catalysis of high temperature and high pressure, oxygen becomes extremely aggressive. It undergoes an electrochemical reaction with iron, forming a very dangerous form of corrosion—pitting corrosion.
Unlike ordinary surface rust, pitting corrosion concentrates its attack on a single point on the metal surface, drilling vertically downwards like a miniature drill. Sometimes, the pipe surface may only show a pinhole-sized rust spot, but in reality, a deep pit has been corroded inside, even penetrating the pipe wall. In addition, dissolved carbon dioxide in the water forms carbonic acid, continuously lowering the pH of the boiler water, keeping the entire system in an acidic corrosive environment. This slow self-destruction is often only discovered during equipment maintenance, but by then it's too late.
The dangers of poor water quality are not limited to the boiler; they travel downstream with the steam flow, ultimately affecting the heart of the power plant—the turbine.
When boiler water contains a large amount of impurities, tiny droplets and dissolved silicates produced during boiling are carried away by the high-pressure steam. When this steam impacts the turbine blades at supersonic speeds, the sudden drop in pressure and temperature causes the dissolved salts (especially silicon compounds) to precipitate instantly, depositing like cement on the high-speed rotating precision blades.
Imagine a massive rotor rotating 3000 times per minute; even a few grams of uneven salt deposits on its blades can cause serious dynamic imbalance. This not only greatly reduces efficiency (meaning burning more coal and generating less electricity), but the violent vibrations can also directly damage the bearings, forcing the power plant to shut down and perform expensive mechanical cleaning.
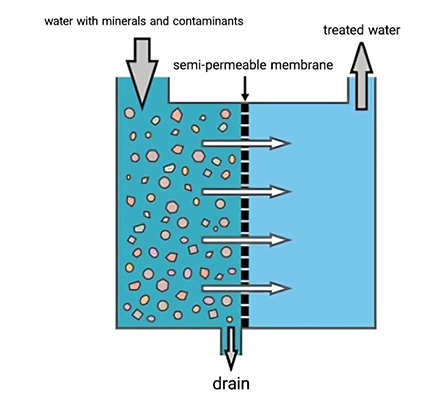
It is precisely to mitigate the risks mentioned above that modern power plants are equipped with large and sophisticated chemical water treatment facilities. This is not merely filtration; it's more like a molecular-level separation process.
We utilize reverse osmosis (RO) technology, using high pressure to force water molecules through a membrane with pore sizes of only nanometers, physically trapping over 98% of ions. Following this, ion exchange resins precisely adsorb the remaining trace amounts of cations and anions, like magnets, releasing H⁺ and OH⁻ to recombine into pure water. Finally, through a combination of thermal deoxygenation and chemical agents, the deadly oxygen in the water is completely removed.
If we compare a power plant to a living organism, the boiler is its heart.
Using ordinary water is like supplying the heart with thick blood containing a large amount of sediment, cholesterol, and toxins. This will not only clog the blood vessels (scaling) and corrode the vessel walls (perforation), but will ultimately lead to a brain (turbine) infarction.
Using ultrapure water, on the other hand, is like injecting the purest, healthiest blood into the system. It ensures efficient heat transfer, unobstructed pipelines, and allows the entire industrial giant to operate safely, economically, and for extended periods.
Therefore, in the world of industrial boilers, choosing ultrapure water is not a matter of choice, but an indispensable safety requirement.