In the field of industrial production, nitrogen oxides (NOx) are a class of pollutants of great concern, especially in the operation of industrial boilers, and their generation and emission should not be ignored.NOx is not only a key indicator of the environmental performance of industrial boilers, but also an important factor affecting the quality of the atmospheric environment. As the core equipment for energy conversion, industrial boilers are widely used in many industries such as chemical, electric power, heating, etc., and they are also one of the main sources of NOx emission.
The large amount of NOx emissions poses a serious threat to the environment and human health. In the environment, NOx is an important precursor for acid rain, photochemical smog and fine particulate matter (PM2.5), which can lead to soil acidification, eutrophication of water bodies, and damage to the ecological balance; in the health level, NOx is a strong irritant, which can lead to respiratory diseases and reduce human immunity, and long-term exposure to high concentrations of NOx may even induce lung diseases and cardiovascular diseases. Long-term exposure to high NOx concentrations may even induce lung and cardiovascular diseases. Therefore, it is of great significance to explore the mechanism of NOx generation in industrial boilers and take effective control measures to realize sustainable industrial development.
NOx is a general term for oxides of nitrogen, mainly composed of nitric oxide (NO) and nitrogen dioxide (NO2). Among them, NO is a colorless and odorless gas at room temperature and pressure, with relatively active chemical properties, easy to react with oxygen to generate NO2; NO2 is reddish brown with irritating odor, a strong oxidant, and will participate in a series of complex chemical reactions in the atmospheric environment.
From the point of view of physical and chemical properties, NO is insoluble in water and easily oxidized in the air; NO2 is soluble in water and reacts with water to produce nitric acid and NO. These characteristics make NOx in the atmosphere have a strong migration and transformation ability, and can be transmitted over long distances to expand the scope of pollution.
In terms of environmental impact, after NOx is emitted into the atmosphere, it will react with volatile organic compounds (VOCs) under sunlight to form ozone (O3), resulting in photochemical pollution; at the same time, NOx will generate nitrate after complex chemical transformation, which is an important component of PM2.5, affecting air quality and jeopardizing human health.
The formation of thermal NOx originates from the chemical reaction between nitrogen (N2) and oxygen (O2) in the air under high temperature conditions. When the temperature in the furnace chamber of an industrial boiler reaches above 1200℃, N2 and O2 molecules get enough energy to dissociate, and the resulting nitrogen atoms (N) and oxygen atoms (O) will rapidly combine to form NO. The reaction process mainly includes: O2 decomposes into O atoms, N2 reacts with O atoms to form NO and N atoms, and N atoms then react with O2 to form NO.
The main factors affecting the formation of thermal NOx include temperature, residence time, and oxygen concentration. The main factors affecting the generation of thermal NOx include temperature, residence time and oxygen concentration. Temperature is the most critical factor, and NO generation increases exponentially with temperature; residence time refers to the length of time the combustion products stay in the high-temperature region, and the longer the residence time, the more NO is generated; and the oxygen concentration has a direct impact on the reaction rate, and a higher oxygen concentration promotes the generation of NO. For the control of thermal NOx, common measures include reducing the flame temperature, such as optimizing the combustion process by using a new type of burner, and staged combustion technology, which reduces the temperature of the local high-temperature region by feeding combustion air into the furnace in stages.
Fuel NOx is generated from nitrogen compounds naturally contained in the fuel. The nitrogen content varies considerably from fuel to fuel, e.g. coal (0.5% - 2%), heavy oil (0.1% - 0.5%), biomass (0.2% - 1%) and natural gas (<0.01%). Nitrogen in fuels can be categorized into volatile and fixed nitrogen. During combustion, nitrogen compounds in fuels are pyrolyzed at high temperatures to volatile nitrogen (e.g., HCN, NH3) or remain in the fuel as solid nitrogen. Volatile nitrogen is oxidized to NO in the oxidation zone, while solid nitrogen may also be oxidized to NO during coke combustion.
The conversion rate of fuel nitrogen is affected by a number of factors, including combustion temperature, oxygen concentration, and combustion time. In order to control the generation of fuel NOx, low nitrogen content fuels can be selected, and staged combustion technology can be used to inhibit the oxidation of nitrogen compounds by lowering the oxygen concentration in the main combustion zone, and completing the combustion by replenishing the air in the burnout zone.
Hydrocarbon radicals" are described as the direct source of NOx generation, but in reality it is the reaction of carbon-based radicals (e.g., CH, C) with nitrogen that generates NO. In industrial boilers, the amount of prompt NOx generation is relatively small, generally accounting for less than 5% of the total NOx. This is because the combustion process in industrial boilers is usually based on the complete combustion of hydrocarbon fuels, and the concentration of hydrocarbon radicals is low, which limits the generation of fast NOx. 4.
Flame temperature is a decisive factor affecting thermal NOx production. As mentioned earlier, high temperatures significantly accelerate the reaction rates of N2 and O2, resulting in a dramatic increase in NOx production. Therefore, effective control of flame temperature is the key to minimizing thermal NOx emissions during the operation of industrial boilers.
The nitrogen content of different fuels directly determines the amount of NOx generated from the fuel. Fuels with high nitrogen content, such as coal and heavy oil, produce more fuel NOx during combustion, while fuels with low nitrogen content, such as natural gas, produce less fuel NOx. In addition, the combustion characteristics of the fuel also affect the NOx generation, such as the volatile matter content and ash characteristics of the fuel.
Oxygen concentration and air excess rate have a significant effect on NOx generation. Higher oxygen concentration promotes the generation of NOx, while a large air excess rate not only increases the flame temperature, but also increases the generation of NOx. Reasonable control of combustion air supply and supply method is an important means to reduce NOx emissions.
The longer the gas residence time in the high temperature region, the more NOx is generated. By optimizing the boiler furnace structure and combustion method, the residence time of the gas in the high-temperature region can be shortened to effectively reduce the generation of NOx.

Low Excess Air Firing: By accurately controlling the amount of air supplied in the combustion process, combustion can be carried out under the conditions close to the theoretical amount of air, lowering the flame temperature and the concentration of oxygen, thus reducing the generation of NOx. However, this method requires strict control of the combustion process to avoid the loss of efficiency and increase in pollutant emissions caused by incomplete combustion.
Staged Combustion: Combustion air is fed into the furnace in stages, with less air supplied to the main combustion zone, so that the fuel is burned under anoxic conditions, reducing the flame temperature and oxygen concentration and suppressing NOx generation; additional air is supplied to the burnout zone to ensure complete combustion of the fuel. Staged combustion technology can effectively reduce heat NOx and fuel NOx emissions.
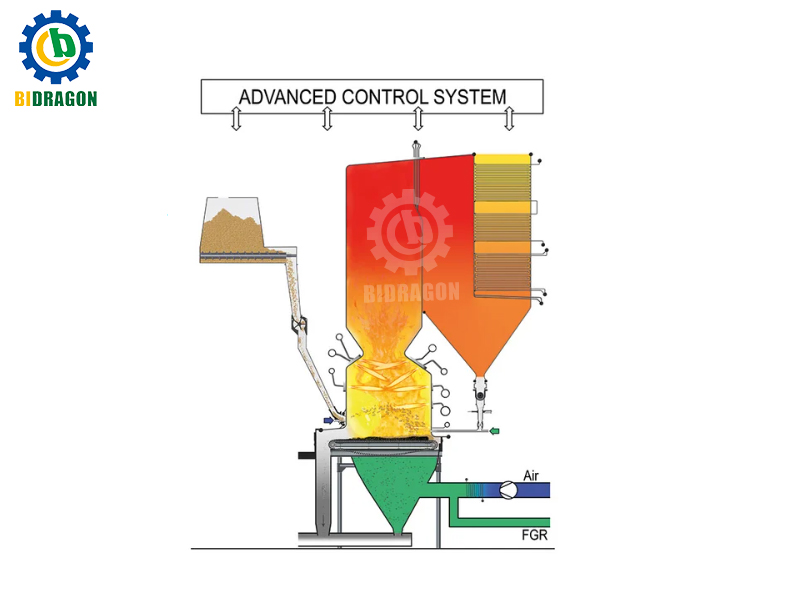
Flue Gas Recirculation (FGR): A portion of the boiler exhaust is reintroduced into the furnace to participate in combustion, lowering the oxygen concentration and flame temperature in the combustion zone and reducing NOx generation. Flue Gas Recirculation (FGR) is usually used in combination with other combustion control techniques to achieve better emission reduction.
Selective Non-Catalytic Reduction (SNCR): A reducing agent such as ammonia (NH3) or urea is injected into the boiler chamber or flue, and under high temperature (850 - 1100℃), the reducing agent reacts with NOx. Under high temperature (850 - 1100℃), the reductant reacts with NOx and reduces it to nitrogen (N2) and water (H2O).SNCR technology has the advantages of low investment cost, easy installation, etc., but the denitrification efficiency is relatively low, generally around 30% - 50%.
Selective Catalytic Reduction (SCR): Under the action of catalyst, the reductant (such as ammonia or urea) reacts with NOx at low temperature (300 - 400℃) to produce N2 and H2O. SCR technology has the advantages of high denitrification efficiency (up to 80% - 95%) and strong adaptability. SCR technology has the advantages of high denitrification efficiency (up to 80% - 95%) and adaptability, but the catalysts are costly and need to be replaced periodically.
Water Injection: Water steam or water is injected into the furnace or flue to reduce NOx generation by lowering the flame temperature and diluting the oxygen concentration. This method is simple to operate, but has a relatively limited effect on NOx reduction.
Choosing the right NOx control method requires a combination of factors such as boiler type, fuel type and application scenario. For example, for small industrial boilers, combustion control technologies such as low excess air combustion and staged combustion can be prioritized to reduce costs; for large power station boilers, due to the higher requirements for NOx emissions, efficient post-combustion control technologies such as SCR can be used. In practical application, a power plant adopts the combination of graded combustion and SCR to reduce the NOx emission concentration from 400mg/m³ to below 50mg/m³, which meets the strict environmental protection emission standards.
The implementation of NOx control measures can have an impact on boiler performance. For example, low excess air combustion may lead to incomplete combustion, reducing boiler efficiency; flue gas recirculation will increase the amount of flue gas, affecting the exhaust temperature of the boiler and induced draft fan power consumption; the use of catalysts in SCR technology may cause catalyst poisoning, clogging and other problems, increasing the maintenance cost. Therefore, when selecting NOx control technology, boiler efficiency, capacity, life and maintenance and other performance parameters must be considered comprehensively to achieve efficient and stable operation of the boiler under the premise of meeting environmental requirements.
In summary, the formation of NOx in industrial boilers mainly consists of three mechanisms: thermal NOx, fuel NOx, and fast NOx, and its generation is influenced by various factors such as flame temperature, fuel type, combustion air characteristics, and residence time. In order to effectively control NOx emissions, combustion control and post-combustion control can be used. In practice, the appropriate NOx control method should be selected according to the specific conditions of the boiler and the relationship between NOx control and boiler performance should be balanced.
Effective NOx management not only helps to reduce air pollution, protect the environment and human health, but also balances the environmental benefits of NOx control with its possible economic costs, and meets the requirements of increasingly stringent environmental regulations. Therefore, industrial enterprises should attach great importance to NOx control, continuously explore and apply advanced NOx reduction technologies, and promote the development of industrial boilers in the direction of green and high efficiency.