In the field of industrial boilers, saturated steam and superheated steam are two extremely important forms of steam. A proper understanding of the differences between the two is essential for optimizing industrial production processes, improving energy efficiency, and ensuring production safety. In this paper, we will discuss saturated steam and superheated steam in depth from the definition, characteristics, differences and applications.
Saturated steam, a state in which water is heated in a furnace by electricity, fuel oil, or other fuels, transforms from a liquid to a gaseous state. In this state, there is a fixed correspondence between the temperature of the steam and the saturated steam pressure, i.e., each saturated steam pressure corresponds to a specific temperature and vice versa.
Wet saturated steam: This is the most common form of saturated steam, which appears as a mixed gas-liquid phase. Wet saturated steam is formed when water is not fully converted to gas during the heating process.
Dry saturated steam: Dry saturated steam has only a gas phase, with no liquid phase present. However, in practice, it is extremely difficult to prepare 100% dry saturated steam. Therefore, wet saturated steam with a dryness greater than 95% (sometimes greater than 98%) is usually considered dry saturated steam. Even the best-performing boilers produce steam that is only 99.8% - 99.9% dry. It is also common to consider slightly superheated steam at temperatures 5 - 40°C above saturation as dry saturated steam, since in this state the heat transfer characteristics of superheated steam are similar to those of dry saturated steam, and at the same time ensures that the steam is “dry,”, there is only a gaseous phase in the steam, with no liquid water present.
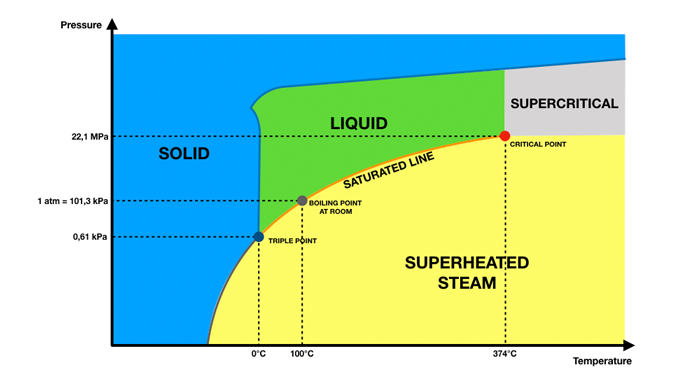
Superheated steam, is a state of steam at a temperature higher than that of saturated steam at the same pressure. The formation process can be understood in this way: the liquid water is continuously heated to a certain temperature, saturated steam will be obtained, at this time the saturated steam may contain the liquid phase, i.e., liquid water, which is known as wet saturated steam; continue to heat until the steam no longer contains the liquid phase, it will be obtained by the dry saturated steam; if the dry saturated steam continues to be heated, the temperature will continue to rise, which will result in the generation of superheated steam.
Theoretically, the temperature of superheated steam can be raised indefinitely until the steam undergoes a change of state and is transformed into a plasma state. However, in actual industrial production, this extreme situation does not occur. In general, the common temperature range of superheated steam is between 380 - 540°C. The temperature of saturated steam is the same as that of superheated steam.
Superheated steam behaves very differently than saturated steam at the same pressure. For example, in the common pressure environment of 1 standard atmosphere, water vapor is saturated at 100°C and is considered saturated steam. Once the temperature of the water vapor exceeds 100°C, it enters the category of superheated steam. This temperature difference is not insignificant. In many industrial processes, the higher temperature of superheated steam can significantly accelerate the rate of chemical reactions and increase productivity, while the fixed temperature characteristics of saturated steam are suitable for basic heating scenarios that do not require a high degree of temperature accuracy.
When the temperature is maintained at a constant level, the pressure characteristics of superheated steam and saturated steam show a clear distinction. In the case of water vapor at 100°C, the pressure of saturated steam at this temperature is 1 standard atmosphere. Superheated steam, on the other hand, has a pressure higher than one standard atmosphere due to its more intense molecular motion and higher internal energy. In some industrial equipment with strict pressure control, this pressure difference determines whether the steam can drive the equipment to operate, such as steam compressors need to be based on the pressure characteristics of the steam to accurately regulate the pressure of the superheated steam, to ensure that the equipment operates efficiently and stably.
Superheated steam has a significant advantage over saturated steam in terms of the amount of heat it carries, a characteristic that is reflected in the specific enthalpy value, which is higher for superheated steam. For example, if you take saturated steam and superheated steam of the same quality and temperature and accurately measure the heat content, you will find that superheated steam contains far more heat than saturated steam. In industrial fields such as metal smelting, where large amounts of thermal energy are required, superheated steam, because of its abundance of heat, can quickly heat metal raw materials to their melting point, greatly reducing smelting time and energy costs.
The temperature and pressure of saturated steam follow a strict correspondence. As long as the pressure is determined, the temperature remains fixed and vice versa. This close correspondence makes saturated steam stable and less prone to sudden changes in specific environments. In contrast, superheated steam has a wider range of temperature and pressure variations and more complex intermolecular interactions. When small changes in external conditions occur, the energy distribution within superheated steam is prone to imbalance, which, in extreme cases, can lead to serious safety accidents such as explosions, for example, in the case of improperly maintained superheated steam piping and abnormally high pressures, which can easily lead to such dangerous conditions.
Superheated steam is fundamentally different from saturated steam in terms of energy transfer. Superheated steam transfers its heat to the object being heated through direct contact with the object, relying on heat conduction, just as a hot iron directly heats the surrounding air. The heat transfer of saturated steam depends on the material phase change process, when saturated steam and lower temperature objects in contact, the steam will be liquefied into liquid water, in this phase change process releases a large amount of latent heat of vaporization, to achieve high efficiency of the heat transfer, the common steam iron is to make use of the principle of saturated steam through the liquefaction of the steam exothermic to smooth the clothing wrinkles.
Based on the characteristics of both, saturated steam and superheated steam have their own focus in the actual application scenarios. Saturated steam is used in scenarios where the temperature and pressure requirements are not too demanding due to its relatively low temperature and pressure. In indoor heating systems, saturated steam can steadily release heat to maintain indoor warmth; in material drying, saturated steam can gently remove moisture from materials to avoid material damage due to overheating; in the field of food cooking, saturated steam can uniformly heat the ingredients to ensure that the food taste and nutrition. By virtue of its high temperature and high pressure characteristics, superheated steam is more suitable for applications that require harsh conditions. In power plant boilers, superheated steam drives turbines to rotate at high speeds, efficiently converting heat energy into electricity; when steam turbines drive large-scale mechanical equipment, the powerful energy output of superheated steam ensures a stable and strong power supply for the equipment.
Saturated steam plays an indispensable role in a wide range of industrial applications. In power plants, saturated steam is used to drive turbines that generate electricity; in heating systems, it provides warmth to buildings; in the food production industry, saturated steam is used for food processing and sterilization; and in chemical manufacturing, saturated steam is an important energy source and reaction medium.
With its versatility and reliability, saturated steam is a key element in countless industrial processes around the world. Its presence guarantees the stable operation of all types of industrial production activities.
Superheated steam has no moisture, is above the boiling point, and has excellent heat-carrying capacity and high energy transfer efficiency. Its ability to achieve precise temperature control makes it ideal for industrial processes where temperature accuracy is critical.
Power generation: When driving a steam turbine, superheated steam is able to achieve better expansion due to its high temperature and pressure, which improves energy extraction efficiency and provides stronger power for power generation.
Drying process: In paper mills, food processing plants and other industries that require drying processes, the moisture-free nature of superheated steam avoids unnecessary condensation and provides a stable and reliable heat source for the drying process.
Equipment Protection: Superheated steam maximizes thermal efficiency and reduces thermal stress on equipment, while reducing the risk of equipment corrosion. In addition, the absence of condensation prevents the occurrence of water hammer, preventing catastrophic damage to piping due to water hammer.
Although both saturated steam and superheated steam originate from the gasification process of water, there are significant differences in definition, characteristics, energy transfer methods, and application scenarios. Saturated steam plays an important role in many low-temperature and low-pressure industrial scenarios due to its stability and applicability, while superheated steam has become the preferred choice for high-temperature and high-pressure industrial applications due to its high-temperature and high-pressure, highly efficient heat transfer characteristics. An in-depth understanding of the difference between the two will help industrial practitioners to rationally select and utilize steam according to actual needs, thereby enhancing production efficiency, reducing production costs and promoting the sustainable development of industrial production. In the future, with the continuous progress of industrial technology, saturated steam and superheated steam will also play a greater value in more areas.